90% OF PRODUCTS IN STOCK
ASSURED QUALITY GUARANTEED
SAME DAY DESPATCH
KG PRICING ON REQUEST
Stock Quantities and re-supply for follow up research.
Typically, about 95% of the compounds in our collection are available in >20mg stock quantities and over 93% of the compounds are available in >100mg stock quantities. Indeed a large proportion of our collection is available in gram quantities; this means we can ensure a very high level of re-supply of originally tested compounds.
Advantages of purchasing directly from BIONET:
- Ease of purchasing directly through our online shop, by phone, or via e-mail
- Usually dispatched within 1 working day, from stock
- Dedicated customer relations department, real-time response
Fragment integrity & formats:
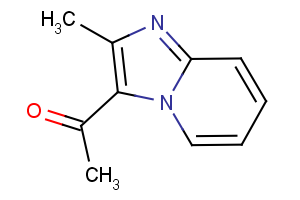
- Minimum Purity of fragments is >90% by LC-MS and/or 1H NMR with the majority being > 95%. Analytical data for every compound purchased can be provided on request.
- Fragment compounds are available in milligram or micromole amounts in powder form plated to your requirements. Bulk amounts available on request
Database Download
To download all the latest BIONET Fragment databases.
Also to receive our updates please register here.