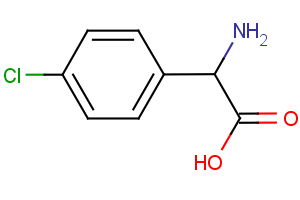
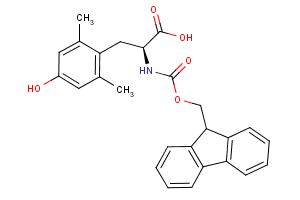
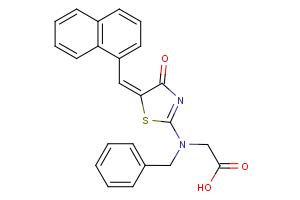
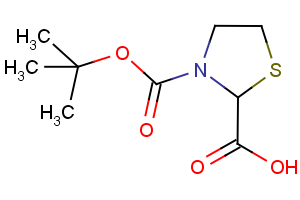
Amino Acids - A unique offering
Over the last decade, the Key Organics BIONET product range has grown from 65,000 to over 342,000 products that are segmented into application and functionality.
Our newly curated collection of Amino Acids contains nearly 7,000 products covering a wide range of unprotected and protected alpha- (α-), beta- (β-), gamma- (γ-)-amino acids (Table 1).
Table 1: Key Organics Amino Acid Collection segmented by structural types and protecting group functionality
Loading..........
The Data is Not Available
These are available in various pack sizes and quantities for both early-stage research and larger scale. Within our chemistry services offerings we can also work exclusively with customers to make novel, targeted products.
Our new process R&D lab allows us to perform scale-up chemistry safely and efficiently on up to 30L scale.
Chemistry Methodologies in Amino Acid Synthesis:
(i) α-Amino Acids
The organic chemist is now able to apply a wealth of new synthetic approaches to amino acids such that there are now an infinite number of novel structures that can be produced at research, development and manufacturing quantities.
The first α-amino acid synthesis was reported by Adolph Strecker in 1850, utilising an aldehyde with cyanide in the presence of ammonia in a condensation reaction that yields an α-aminonitrile (Figure 1). This is subsequently hydrolysed to give the desired α-amino acid as a racemic mixture.
Figure 1: The Strecker Amino Acid Synthesis

Catalytic asymmetric Strecker reactions can also now be achieved using thiourea-derived catalysts. In 2012, a BINOL derived catalyst was employed to generate chiral cyanide anion and subsequently the homochiral α-amino acid (Figure 2).1
Figure 2: Scalable organocatalytic asymmetric Strecker reaction

Through additional innovations in biocatalysis, asymmetric catalysis and the resolution and recycling of racemic mixtures, medicinal and process development chemists now have a wide range of technologies to make and produce an infinite range of novel homochiral amino acids.
Amino Acids represent an important elaboration of our BIONET offerings given increasing and innovative R&D in new peptide-based therapeutics and as exemplified above, access to new methodologies for their synthesis and large-scale production.
- Yan, H., Suk Oh, J., Lee, JW. et al. Scalable organocatalytic asymmetric Strecker reactions catalysed by a chiral cyanide generator. Nat Commun 3, 1212 (2012)
- Lelais, G. and Seebach, D. (2004), β2-amino acids—syntheses, occurrence in natural products, and components of β-peptides1,2. Biopolymers, 76: 206-243
- Enantioselective Synthesis of β-amino acids: A Review. Ashfaq et al. Med chem 2015, 5:7
- Wang, LC., Yuan, Y., Zhang, Y. et al. Cobalt-catalyzed aminoalkylative carbonylation of alkenes toward direct synthesis of γ-amino acid derivatives and peptides. Nat Commun 14, 7439 (2023).
(ii) β-Amino Acids
β-amino acids differ by a methylene unit from their α-homologues and have specific applications in peptidomimetics, where substituting α-amino acid residues for β-amino acids can alter the biological properties of synthetic peptides by improving metabolic stability and target selectivity compared to the parent peptide.2 Efficient synthetic access to β-amino acids is also desirable due to the prevalence of the moiety in natural products and drugs.
Ashfaq et al. have published an excellent review of current synthetic methodologies to this class.3 While more than 80 different synthetic strategies are discussed in their review, the organo-Rh based and heterogeneous catalysed approaches are found to be most effective (eg Figure 3).
Figure 3: Asymmetric Synthesis of β-Amino Acids using Rh(bisphosphine) Catalysis

We have access to both known and novel chiral bisphosphine ligands such that we can undertake catalyst screens on specific/fixed substances. Synthetic and cost effective access to the substrate is typically the limiting factor in this approach, particularly with respect to large development and manufacturing quantities.
(iii) γ-Amino acids
γ-Amino acids have attracted considerable attention as biologically active compounds. In recent years interest in the stereoselective synthesis and practical application of linear and cyclic chiral γ-amino acids in the synthesis and design of α,β- and β,γ-hybrid peptides with definite secondary structures and design of nanotubes has been reported, underpinning both the theoretical and practical importance of this class.
γ-Amino acid synthesis generally involves chemical methods like hydrolysis of γ-lactams or Michael-type addition, or more recently, transition metal-catalysed carbonylation. However, these methods are often multi-step and can have limited substrate scope, impacting efficiency. Enzymatic approaches, like those involving aldol addition and transamination, are also being explored for the synthesis of γ-hydroxy-α-amino acids.
In their publication in the journal Nature Communications, Wu and co-workers provide a very comprehensive review of current methodologies in which they discuss three specific strategies (Figure 4).4
Figure 4.
Strategy I

Michael-type addition
scope limiting
Strategy II

Activated Michael donor & acceptor
difficult to modify
Strategy III

Hydrolysis reaction
single structure & scope limiting
Compared with the well-established synthesis of α- and β-amino acids, general catalytic approaches to afford γ-amino acids are relatively underdeveloped. Indeed, the number of commercially available γ-amino acids is mainly limited to simple and natural amino acids, while most other γ-amino acids require complex synthetic procedures. Current approaches mainly rely on the hydrolysis of the corresponding γ-lactam (or γ-butyrolactone) or the Michael-type addition of carbonyl and nitro compounds. These traditional synthetic routes towards γ-amino acids are limited by multistep preparation and narrow substrate scope, which seriously reduces the efficiency of the synthesis process (Figure 4). Transition metal-catalysed carbonylation represents one of the most effective and powerful strategies for the installation of carbonyl groups.
Benefits of our Collections:
Re-supply and Bulk supply for follow up research.
A large proportion of our collection is available in bulk quantities; this means we can ensure a very high level of re-supply to help your project succeed.
And if you want to explore analogues or some initial Process Development Chemistry, our specialist team would love to help.
Advantages of purchasing directly from BIONET:
- Ease of purchasing directly through our online shop, by phone, or via e-mail
- Usually dispatched from stock same day
- We maintain good stock levels
- Dedicated customer relations department
Database Download:
To download all the latest BIONET databases, in SD and MS Excel file formats